Which of the following best describes a weak base. A base that dissociates very slightly in a water solution 18.

Chemistry Final Exam Sample Items Which Idea Of John Dalton Is No
Vinegar fruit juice and cola are examples of.

. Hence ammonium ion is present. Which of the following best describes this solution. An antacid increases the amount of hydrogen ions.
A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. H acceptor C acidic. H acceptor C acidic.
Positive ions have more electrons than neutrons. Then a solution that has a pH of 12 is a very strong base. 1 mol H ions 60221023 ions.
Which of the following best describes this solution. Which of the following best describes this solution. This corresponds to a concentration of 10-7 mol H ions per litre of water.
The gas is carbon dioxide. Something can be acidic basic or neutral. H acceptor B basic.
H acceptor B acidic. A solution that contains equal numbers of hydrogen and hydroxyl ions would be called acidic basic alkaline neutral was asked on May 31 2017. View solution Which of the following statement is not correct for the element having electronic configuration 1 s 2 2 s.
A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. H donor D basic. PH of more than 7 is basic.
If you want to know the actual number of H ions in the litre of water. A solution contains 00000001 10-7 moles of hydrogen ions H per liter. 32 A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter.
Positive ions have more protons than neutrons. H donor D neutral Answer. Which of the following best describes this solution.
H acceptor B basic. If the buffer is composed of HA and A and a strong acid eg HCl is added the buffer absorbs hydrogen ions in the following way. A solution is defined as acidic if the concentration of hydrogen ions is greater than the concentration of hydroxide ions.
H acceptor QUESTION 4 In pure water H and OH- concentrations are equal. Q contains a carbonate ion. And OH - is the concentration of hydroxide ions in solution.
Up to 24 cash back d. Write the symbol for the ion that has 46 electrons and has lost 4 electrons. The solution will have a pH that is more than 7 17.
H OH - where H concentration of hydrogen ions in solution. A typical acidic solution has H present in solution. A buffer prevents the pH of a solution from changing when an acid or base is added.
Salt P is ammonium chloride. A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter. H donor E neutral A solution contains 10-3.
There is also 10-7 mol OH- ions per litre of water. H acceptor C acidic. A dilute strong base d.
H donor neutral A solution contains 00000001 10-7 moles of hydrogen ions H per liter. Click Save and Submit to save and submit. Which of the following best describes this solution.
Figure shows the reaction scheme of a compound Q. The best term to describe this mixture would be _____ an aqueous solution If the molecular mass of a carbon atom is 12 the mass of a hydrogen atom is 1 and the mass of an oxygen atom is 16 daltons how many molecules does one mole of table sugar sucrose. The scale of pH goes from extremely acidic at 1 to neutral at 7 to extremely basic at 14.
Anion is a chloride ion because silver chloride is precipitated. HA NaOH NaA H₂O. An example would be Ammonia.
Which statement describes positive ions. An antacid decreases the level of hydrogen ions. QA Biology A solution that contains equal numbers of hydrogen and hydroxyl ions would be called acidic basic alkaline neutral.
H donor D neutral. What do hydrogen ions. H OH -.
View the answer now. Buffered solutions are always neutral with a pH of 7. What determines if a solution is neutral is the ions present in solution.
A buffer prevents the pH of a solution from changing when an acid or base is added. A base with a very low concentration b. Polar QUESTION 3 A solution contains 0000000110-7 moles of hydroxyl ions OH- per liter.
The gas liberated is ammonia because it is an alkaline gas. Which of the following best describes this solution. A base that is not very strong c.
An antacid neutralizes excess hydrogen ions. How does the addition of an acid affect a neutral solution the h ion concentration is increased a given solution contains 000011 10-4 moles of hydrogen ions h per liter. Note that this MOLES of H or OH- ions per litre of water.
Which statement best describes an antacid. Part A Select the statement that best describes a buffer. Give the number of protons and electrons in S2-.
Buffer resists change in pH by accepting ttydrogen ions when acids are added to the solution and donating. Which of the following best describes the term end point. Which of the following best describes this solution.
PH of less than 7 is acidic. A HCl HA Cl If a solid base eg NaOH is added the buffer grants hydrogen ions in the following way.

Neutral Solution Definition Examples Video Lesson Transcript Study Com
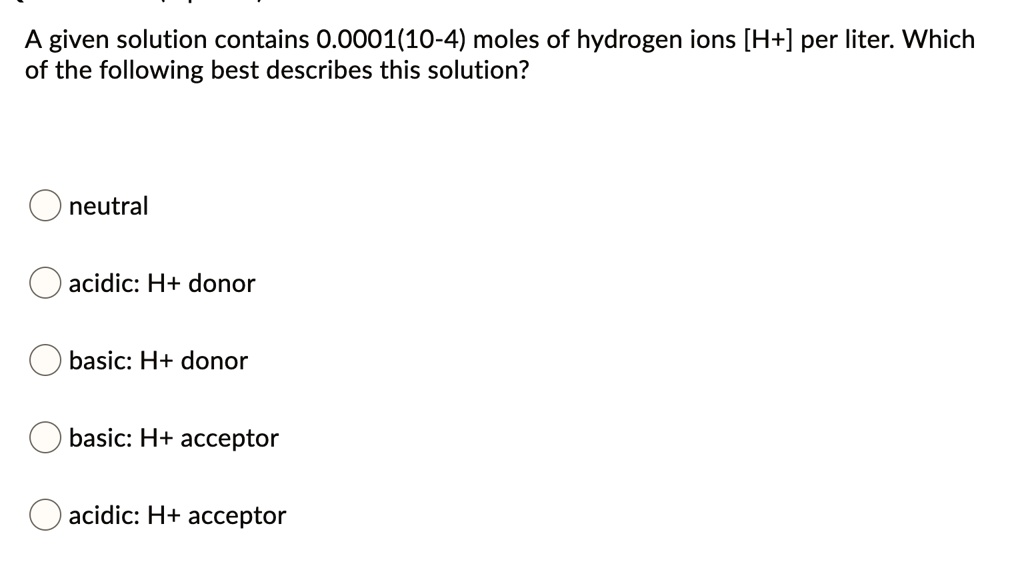
Solved A Given Solution Contains 0 0001 10 4 Moles Of Hydrogen Ions H Per Liter Which Of The Following Best Describes This Solution Neutral Acidic H Donor Basic H Donor Basic H Acceptor Acidic H
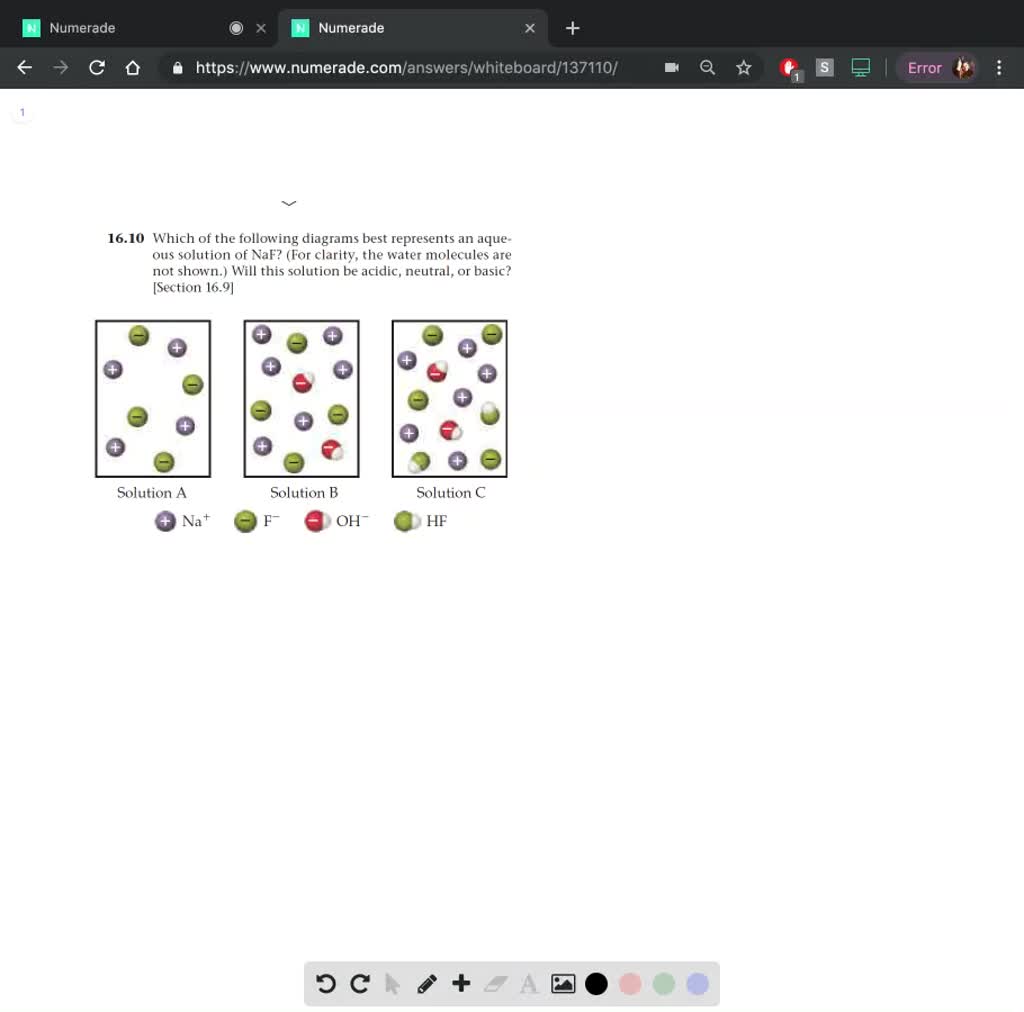
Solved Which Of The Following Diagrams Best Represents An Aque Ous Solution Of Naf For Clarity The Water Molecules Are Not Shown Will This Solution Be Acidic Neutral Or Basic Section 16 9
0 Comments